THE CHALLENGE
Cesium-137 is a radioactive isotope which is a common fission product of nuclear reactors around the world. After nuclear accidents, Cs is easily spread throughout the environment via the air and is extremely difficult to remove from the environment due to water-solubility. High levels of exposure to Cs-137 can lead to death, while lower levels can lead to elevated risks of disease such as cancer. There are currently no efficient ways to remove Cs from contaminated sites.
OUR SOLUTION
The laboratory of Amanda Morris at Virginia Tech has developed novel Aluminum-based molecular cages. The cages are insoluble and capable of binding dissolved Cs+ ions in solution. Importantly, the structure of these cages allows for a high specificity to Cs+ binding over other dissolved ions with similar chemical properties such as K+, presenting a promising solution for selective Cs removal from environments.
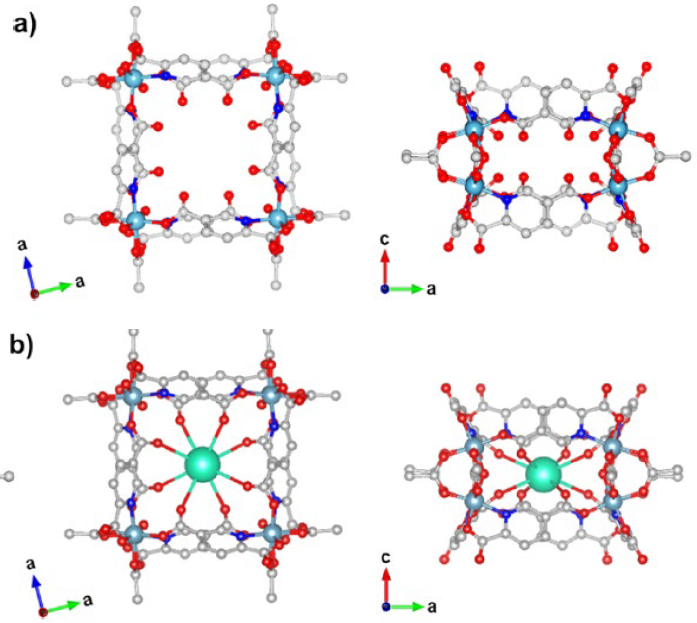
Figure: Crystal structure of the molecular cage obtained from Single-Crystal X-Ray Diffraction (SCXRD) with (a) and without (b) a complexed Cs+ ion.