THE CHALLENGE
Plasmonic nanostructures have enabled label-free surfaceenhanced Raman spectroscopy (SERS) to emerge as a rapid nondestructive molecular fingerprint characterization technique for complex biological samples. However, label-free SERs bioanalysis faces significant challenges in reliability and reproducibility due to local optical field variations at plasmonic hotspots.
OUR SOLUTION
The Zhou lab has shown that plasmonically enhanced electronic Raman scattering (ERS) signals from metal nanostructures can serve as a SERS calibration internal standard to improve multivariate analysis of living biological systems. ERS-based SERS calibration can enhance supervised learning classification of label-free living cell SERS spectra in (1) subtyping breast cancer cells with different degrees of malignancy and (2) assessing cancer cells' drug responses at different dosages.
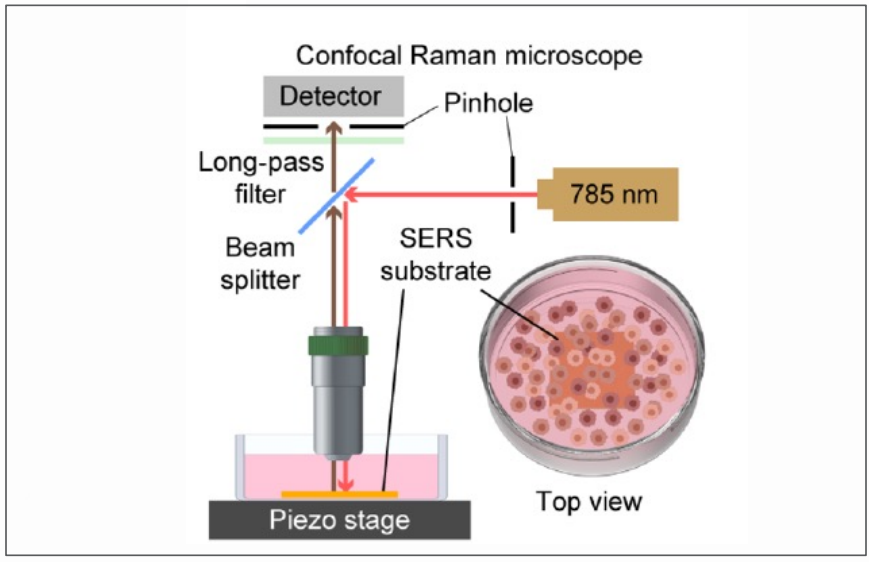
Figure: A scheme for a 2D label-free SERS measurement of living breast normal cells and cancer cells cultures on the nanolaminated SERS substrates.