THE CHALLENGE
Tobacco smoking is one of the largest public health threats the world has ever faced and results in over seven million deaths annually world wide. Smoking cessation is a difficult and largely unsuccessful undertaking; only 3-5% of unassisted smokers are able to remain abstinent for more than six months, and the use of pharmacological inventions—including nicotine replacement therapy—only raises that success rate to 10-25%.
OUR SOLUTION
Chenming Zhang his team at Virginia Tech chose to approach the smoking cessation challenge a different way; they have developed a nanoparticle-based nicotine nanovaccine. This new nanovaccine induces the production of antibodies that recognize and bind to nicotine. Once that binding occurs, the antibody-nicotine complex is too large to cross the blood brain barrier so nicotine can’t trigger the neurological response that makes smoking pleasurable. Preliminary studies in mice indicate that this nanovaccine can block up to 67% of nicotine from reaching the brain. The vaccine is also safe, highly immunogenic, and appropriately bioavailable due to PEGylation. These findings indicate that this approach is a promising method of fighting nicotine addiction.
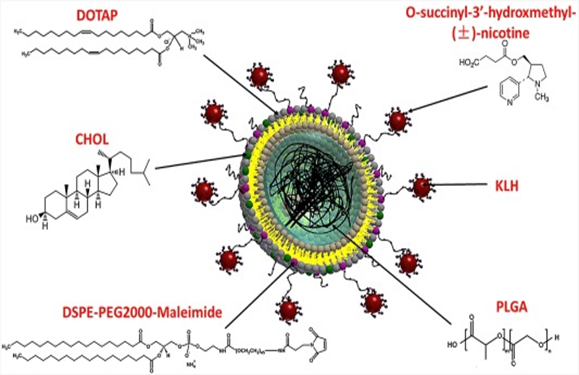
Figure: Schematic illustration of the structure of nanovaccine nanoparticles. Poly(lactic-co-glycolic acid) (PLGA) nanoparticles serve as a scaffold that is capable of supporting the outside lipid layer and stabilizing the vaccine delivery system. The lipid-maleimide component of the envelope layer enables the association of carrier protein (KLH or CRM197) onto the surface of lipid-PLGA nanoparticles. Nicotine-haptens are conjugated to KLH or CRM197 to be immunogenic.